
Speed up Your Breakthroughs with Precision Instruments
Knowledge is power. Leverage our OEM expertise and in-depth knowledge of the life sciences industry to streamline your module manufacturing processes. Our specialists understand the complexities involved and can help you address critical challenges as you prepare for manufacturing.
What We Offer:
Integrate relevant standards and regulations into your manufacturing process.
Fine-tune your approach to optimize productivity and reduce costs.
Enhance manufacturing techniques to produce high-value products.


At ACHB, we recognize that efficient designs drive success in life science equipment.
Improve existing designs for optimized functionality.
Solve design challenges to ensure high performance and reliability.
Provide extensive analytical support to select the right manufacturing processes for complex designs.

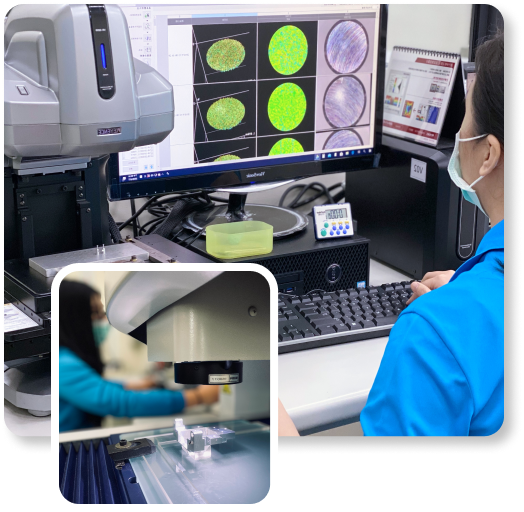


Validating your proof of concept (PoC), alpha, or beta prototype before market launch is crucial for success. Our decades of experience in the life sciences and medical device markets give us the expertise needed to alleviate your concerns. ACHB employs an ISO 27001-certified process combined with our extensive knowledge to test products and ensure standardized quality.
Rigorous quality control services guarantee
Reliable performance of life science modules, systems, and components.
Application of device-specific regulations to verify product quality.
Access to industry experts to address complex manufacturing and design challenges.
Science Moves Forward... Will You?
Our extensive experience in manufacturing medical devices and laboratory equipment serves as a strong foundation for producing critical modules for life science applications. ACHB showcases its expertise across various module types.
We specialize in crafting essential fluid modules for life science equipment, designing fluidic systems that align with your equipment's specifications.
Our offerings include:
- Valve Systems
- Pumping Systems
Our module-making expertise extends to intelligent detection systems for life science applications.
Create detection modules for:
- Detector Systems
- Sensory Systems
Our robotics and system engineering teams excel in manufacturing intelligent automation modules, enhancing life science instruments and automated equipment.
Create automation modules for:
- Robotic Arms
- Automated Systems
- Sample Handlers
- Laboratory Equipment
With extensive support to manufacturers in the medical device, laboratory, and IoT sectors, our specialists design and create temperature control modules tailored to your requirements.
Enhance your systems with expertise in:
- Heating and Cooling Systems
- Temperature Measurement Systems
- Monitoring Systems
Our stellar reputation in supporting OEMs in the life sciences industry underscores our design expertise.
Here’s What Our Clients Say:



Discover New Frontiers with ACHB
With over 30 years of combined experience in the life science, medical device, and laboratory equipment manufacturing sectors, our team of expert engineers and industrial designers understands the regulatory landscape. We apply these standards to ensure your project’s success. Our facilities are ISO 13485-certified, providing the assurance you need for reliable, high-quality products.
Let's transform your ideas into reality. For innovative, reliable manufacturing solutions tailored to your needs.


